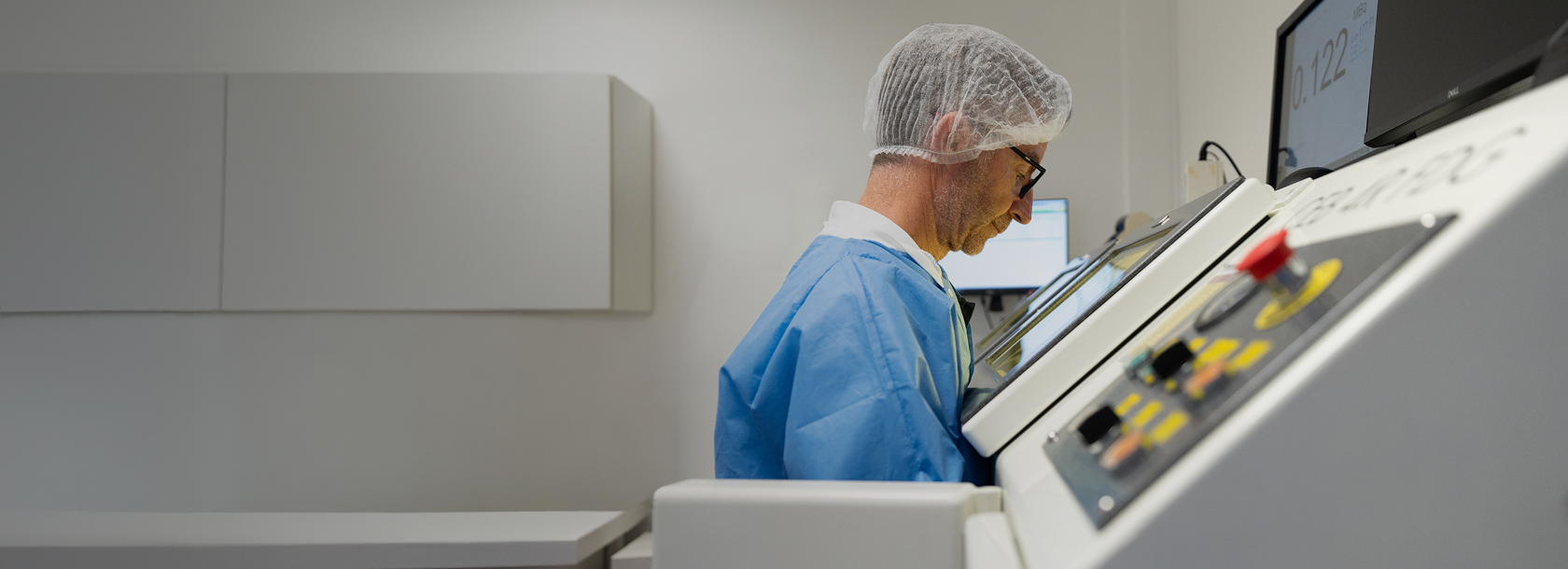
Review your center readiness for RLI & RLT




This site is intended for healthcare professionals outside of the United States (US) and the United Kingdom (UK).
This site is created and funded by Novartis.
If you are an HCP from one of the countries listed, you may select your country from the list to be redirected to your specific site.







Created in collaboration with industry experts, the questions below have been designed to help you understand your center’s current status and identify any areas where support or development may be beneficial. These questions should be viewed as a non-directive reference tool only, to help you reflect on your RLI and RLT readiness.
You can download these questionnaires here:
These questionnaires are intended to support consistent, transparent information-gathering and to serve as a reference for discussion, reflection, and documentation.
Items are prompts to consider, not required actions. The questionnaires are non-prescriptive and should not be interpreted as mandates, rules, or exhaustive checklists.
Users should adapt or skip questions based on context, organizational policies, and applicable laws and regulations.
No warranty is expressed or implied regarding completeness, accuracy, or fitness for a particular purpose; use is at the user’s discretion and risk.
Users are responsible for ensuring alignment with local policies, guidelines, regulations and laws. The content is for general informational purposes only and may not reflect all current best practices or regulations.
Topics cover facility layout, radiation safety, imaging procedures, patient & drug management, and the importance of multi-disciplinary tumor board meetings.
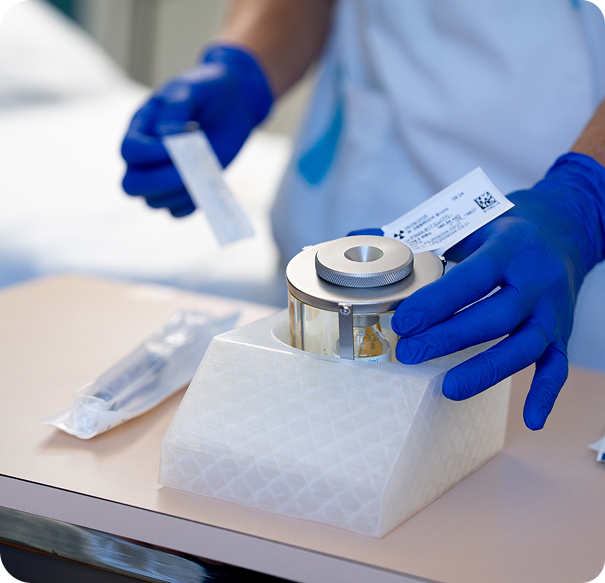
Our dedicated RLT specialist teams aim to streamline care coordination and alleviate uncertainty, ensuring you and your patients feel confident and supported every step of the way.